Basic knowledge related to LNG (I)
Liquefied natural gas (LNG) has become one of the main gas sources for cities that cannot use pipeline natural gas. It is also a supplementary gas source or peak-shaving gas source for many cities that use pipeline natural gas. This article mainly introduces the basic knowledge related to liquefied natural gas, from the process of receiving LNG to gasification, the main equipment commonly used in LNG gasification stations, operation and maintenance, and emergency repairs.
Section 1 Basic Knowledge of Liquefied Natural Gas
When natural gas is cooled to about -162℃ under normal pressure, it changes from gas to liquid, which is called liquefied natural gas (LNG). The main components of LNG are methane, as well as small amounts of ethane, propane and nitrogen.
Natural gas is further purified during the liquefaction process, and the methane is of higher purity, contains almost no carbon dioxide and sulfide, and is colorless, odorless and non-toxic.
1. Nature of LNG
1. Density
The density of LNG depends on its composition and temperature, usually between 430 kg/m3 and 470 kg/m3, but in some cases it can be as high as 520 kg/m3. The density changes with temperature by about 1.35 kg/(m3·℃). The volume of LNG is about 1/600 of the volume of the same amount of natural gas in gaseous form.
(2) Boiling point
Boiling is the phenomenon that the liquid vaporizes both inside and on the surface at a certain temperature and pressure. The temperature at which the liquid boils is called the boiling point. The boiling point of LNG depends on its composition and pressure, and is usually between -166°C and -157°C at normal pressure.
(III) Evaporation of LNG
LNG is stored in an insulated tank in a boiling state. Any heat transferred to the tank will cause part of the LNG to evaporate into gas, which is called boil-off gas. Its composition is related to the composition of LNG. When LNG evaporates, nitrogen and methane are the first to be vaporized from the liquid due to their lower boiling points. Generally, boil-off gas includes about 20% nitrogen, 80% methane and a trace amount of ethane. The nitrogen content of boil-off gas is 20 times that of LNG.
(IV) Flash evaporation
When liquid is heated in a closed container, the pressure of the gas phase continues to increase due to the evaporation of the liquid phase. When the liquid and gas reach a state of equilibrium, if the gas phase of the container is suddenly connected to a low-pressure external environment, the gas phase pressure will immediately drop, the liquid will boil rapidly, and the phenomenon of a large amount of liquid evaporating into the gas phase is called flash evaporation.
Flashing also occurs when the LNG pressure in a container or pipeline suddenly drops below its saturated vapor pressure. Since LNG is a mixture of multiple components, the components of the flash gas are different from those of the remaining liquid. As a guideline, when the pressure is 100kPa to 200kPa, 1m3 of liquid produces approximately 0.4kg of gas for every 1kPa drop in pressure.
5. LNG spillage, expansion and diffusion
When LNG is dumped onto the ground (e.g., an accidental spill), it will initially boil violently, and then the evaporation rate will rapidly decay to a fixed value that depends on the thermal properties of the ground and the heating of the surrounding air. When the spill occurs over water, the convection in the water is strong enough to keep the evaporation rate constant within the area involved, and the spill area of LNG will continue to expand until the total amount of gas evaporated equals the total amount of LNG leaked.
Initially, the temperature of the boil-off gas is almost the same as that of the LNG, and its density is greater than that of the surrounding air. The gas first flows along a layer above the ground until it absorbs heat from the atmosphere and warms up. When the temperature of the LNG is -107°C, its density is close to that of the air, and as the temperature continues to rise, its density will become less than that of the surrounding air.
Following the spill, a "fog" cloud will be produced due to the condensation of water vapor in the atmosphere. When this "fog" cloud is visible (in the daytime and without natural fog), this visible "fog" cloud can be used to show the movement of the evaporating gas and give a conservative indication of the flammability range of the gas-air mixture. In the event of a spill in a pressure vessel or pipeline, LNG will enter the atmosphere in the form of a jet stream, and will expand and evaporate at the same time. This process occurs simultaneously with intense mixing with the air. Most of the LNG is initially contained in the gas cloud in the form of an aerosol (a colloidal dispersion system formed by small solid or liquid particles dispersed and suspended in a gas medium). This aerosol will eventually evaporate by further mixing with the air.
(VI) LNG pool fire
For a burning LNG pool with a diameter greater than 10m, the surface radiation power of the flame is very high (i.e. the temperature is very high). The surface radiation power depends on the size of the fire pool, the dispersion of the smoke and the measurement method. The surface radiation power decreases with the increase of soot and carbon black.
(VII) Tumbling
Rollover refers to the process in which a large amount of gas is released from an LNG container in a short period of time. Unless preventive measures are taken or the container is specially designed, rollover will cause the container to overpressurize or be destroyed.
As heat is input into the container, heat and mass transfer between units and evaporation of the liquid surface occur. The density between units will reach equilibrium and eventually mix into one. This spontaneous mixing is called tumbling.
8. Phase Transition
When two liquids at different temperatures come into contact under certain conditions, a phase change sometimes occurs that can produce explosive forces. This phenomenon, called a rapid phase change, occurs when LNG comes into contact with water. Although combustion does not occur, the phenomenon has all the other characteristics of an explosion.
9. Boiling liquid expansion and vapor explosion
Any liquid at or near its boiling temperature and under pressure above a certain value, if suddenly released due to failure of the pressure system, will evaporate at an extremely high rate. This phenomenon is called boiling liquid expansion vapor explosion.
2. Liquefaction of Natural Gas
1. Natural gas pretreatment
As the raw gas for the liquefaction unit, natural gas must first be pre-treated. Natural gas pre-treatment refers to the removal of impurities such as hydrogen sulfide, carbon dioxide, moisture, heavy hydrocarbons and mercury in natural gas to prevent these impurities from corroding equipment or freezing at low temperatures and clogging equipment and pipelines.
(II) Natural Gas Liquefaction Process
There are different forms of natural gas liquefaction process, which can be divided into the following three methods according to the refrigeration method:
1. Cascade liquefaction process
2. Mixed refrigerant liquefaction process
3. Liquefaction process with expander
Such a division is not strict, and a composite process that includes different combinations of certain parts of the above-mentioned liquefaction processes is usually adopted.
3. Introduction to Natural Gas Refrigeration Process
Common natural gas liquefaction refrigeration processes include cascade refrigeration process, mixed refrigeration process and expansion refrigeration process.
1. Cascade refrigeration process
The cascade refrigeration process is a conventional refrigeration process. For the natural gas liquefaction process, it is generally composed of three refrigeration cycle stages with propane, ethylene and methane as refrigerants, which provide the cooling capacity required for natural gas liquefaction step by step, and the refrigeration temperature gradients are about -30 ℃, -90 ℃ and -150 ℃ respectively. The purified raw natural gas is cooled, condensed, liquefied and supercooled step by step in the coolers of the three refrigeration cycles. After throttling and reducing the pressure, a low-temperature and normal-pressure liquefied natural gas product is obtained and sent to the storage tank for storage.
The refrigeration system and the natural gas liquefaction system of the cascade refrigeration process are independent of each other, the refrigerant is a single component, the systems have little mutual influence, the operation is stable, and it is more suitable for high-pressure gas sources (using gas source pressure energy). However, due to the large number of refrigeration units and long process, the refrigerant purity requirements are strict, and it is not suitable for natural gas with a high nitrogen content. Therefore, this liquefaction process has been rarely used in natural gas liquefaction equipment.
(II) Hybrid Refrigeration Process
The mixed refrigeration process evolved from the cascade refrigeration process in the late 1960s. It mostly uses hydrocarbon mixtures (N2, C1, C2, C3, C4, C5) as refrigerants to replace multiple pure components in the cascade refrigeration process. The refrigerant composition is determined by the composition and pressure of the raw gas. The heavy components in the multi-component mixture condense first and the light components condense later. The mixtures are condensed, separated, throttled, and evaporated in sequence to obtain cooling capacity at different temperature levels. Depending on whether the mixed refrigerant is mixed with the raw natural gas, there are two types of mixed refrigeration processes: closed and open.
Closed cycle: The refrigerant circulation system is an independent system. After the mixed refrigerant is compressed by the refrigeration compressor, it is cooled by water (air) and condensed and separated step by step at different temperatures. After throttling, it enters the different temperature sections of the cold box (heat exchanger) to provide cooling capacity for the raw natural gas. After the raw natural gas is treated with "three removals", it enters the cold box (heat exchanger) and is cooled, condensed, throttled, and depressurized step by step to obtain the liquefied natural gas product.
Open cycle: The raw natural gas is mixed with the mixed refrigerant after being treated with "three degassings" and then flows through various levels of heat exchangers and gas-liquid separators in sequence. While gradually condensing, the required refrigerant components are also condensed and separated one by one. The separated refrigerant components are evaporated step by step according to the boiling point of the refrigerant, and gathered to form a low-temperature logistics, which is a refrigeration cycle of countercurrent heat exchange with the raw natural gas. The open cycle system takes a long time to start up, is difficult to operate, and the technology is not yet perfect.
Section 2 LNG Station Process Flow
There are two common types of LNG stations: LNG gasification stations and LNG bottle group gasification stations. LNG gasification stations refer to stations that have the functions of unloading, storing, gasifying, regulating pressure, metering and odorizing LNG transported by tank trucks or tank ships, and sending it to urban gas transmission and distribution pipelines. They are often used in cities where pipeline gas cannot be used. LNG bottle group gasification stations refer to those that use gas cylinder groups as storage and gas supply facilities. Their gas supply scale is small and they are mainly used for gas supply to communities or individual industrial users. This section focuses on a detailed analysis of the process flow of gasification stations and briefly introduces the process flow of bottle group stations.
1. LNG gasification station process flow
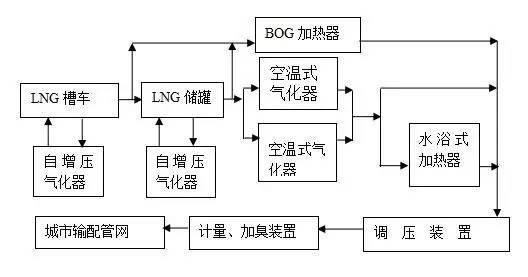
Figure 1 LNG gasification station process flow
LNG gasification station is the main mode adopted in downstream LNG application, and its main function is to store and gasify LNG. It includes unloading platform, cryogenic storage tank, boosting system, gasification system, pressure regulating, metering and odorization system. This section focuses on the unloading process, self-pressurization process of storage tank and gasification heating process of LNG gasification station, as well as the attached BOG and EAG processes. The process flow of common LNG gasification station is shown in Figure 1.
1. LNG unloading process
LNG is transported from LNG liquefaction plants and marine receiving terminals to LNG gasification stations in gas-consuming cities by road tankers or tank container trucks, and is weighed and measured by truck scales. The tank truck is connected to the corresponding pipelines of the unloading platform with a metal hose, and the tank truck is pressurized by the unloading booster gasifier in the station to form a certain pressure difference between the tank truck and the LNG storage tank. This pressure difference is used to unload the LNG in the tank truck into the storage tank of the gasification station. At the end of unloading, the gas phase natural gas in the tank truck is recovered through the gas phase (BOG) pipeline of the unloading platform, as shown in Figure 2.
When unloading, different unloading methods are used to prevent the pressure in the LNG storage tank from increasing and affecting the unloading speed. When the temperature of the LNG in the tank truck is lower than that of the LNG in the storage tank, the upper liquid inlet method is used. The low-temperature LNG in the tank truck enters the storage tank through the nozzle of the upper liquid inlet pipe of the storage tank in a spraying state, cooling part of the gas into liquid and reducing the pressure in the tank, so that unloading can proceed smoothly. If the temperature of the LNG in the tank truck is higher than that of the LNG in the storage tank, the lower liquid inlet method is used, and the high-temperature LNG enters the storage tank from the lower liquid inlet, mixes with the low-temperature LNG in the tank and cools down, avoiding the high-temperature LNG from entering the tank from the upper liquid inlet and evaporating, which increases the pressure in the tank and causes difficulty in unloading.
In actual operation, since the LNG gas source is currently far away from the gas-consuming city, when it reaches the gas-consuming city after long-distance transportation, the temperature of the LNG in the tank truck is usually higher than the temperature of the LNG in the gasification station storage tank, so the bottom liquid inlet method is adopted.
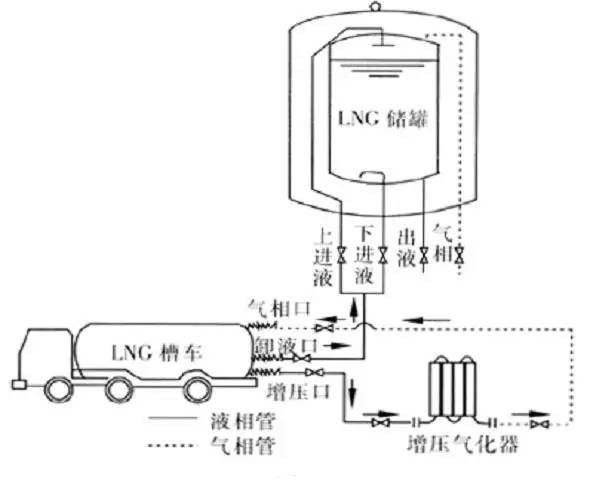
Figure 2 Pressure-boosting unloading process
(II) Automatic pressurization process of storage tank
As the LNG in the storage tank continuously flows out to the vaporizer, the pressure in the tank continues to decrease, and the speed of LNG out of the tank gradually slows down until it stops. Therefore, in normal gas supply operation, gas must be continuously added to the storage tank to maintain the pressure in the tank within a certain range in order to keep the LNG gasification process going. The pressurization of the storage tank is achieved by using an automatic booster regulating valve and a self-pressurized air-temperature vaporizer. When the pressure in the storage tank is lower than the set opening value of the automatic booster valve, the automatic booster valve opens, and the LNG in the storage tank flows into the self-pressurized air-temperature vaporizer by the liquid level difference. In the self-pressurized air-temperature vaporizer, LNG is gasified into gaseous natural gas through heat exchange with air, and then the gaseous natural gas flows into the storage tank, raising the pressure in the storage tank to the required working pressure. During the self-pressurization process, as the gaseous natural gas continues to flow in, the pressure of the storage tank continues to increase. When the pressure rises to the closing pressure of the automatic booster regulating valve, the automatic booster valve closes and the pressurization process ends. As the gasification process continues, when the pressure in the storage tank is lower than the opening pressure set by the booster valve, the automatic booster valve opens and a new round of boosting begins.
(III) LNG gasification and heating process
When LNG flows from the storage tank to the air-temperature vaporizer and is vaporized into gas, it is greatly affected by the ambient temperature. In summer, the outlet temperature of the air-temperature vaporizer can reach above 15°C and can be directly used in the pipeline network. In winter or rainy season, the vaporization efficiency of the vaporizer is greatly reduced, especially in the cold north. In winter, the temperature of the natural gas at the outlet of the vaporizer (about 10°C lower than the ambient temperature) is far below 0°C and becomes low-temperature natural gas. The gasified natural gas needs to be heated to above 10°C by a water bath heater before being sent to the urban transmission and distribution pipeline network.
Usually, two groups of air-temperature vaporizers are set up and used alternately. When one group is used for too long and the vaporizer is severely frosted, resulting in reduced vaporization efficiency and outlet temperature that does not meet the requirements, it is manually (or automatically or timed) switched to another group for use, and this group is used for natural defrosting and standby.
(IV) Natural evaporation process
BOG is the abbreviation of B0il Off Gas, which means naturally evaporated natural gas. During the storage of LNG in the tank and the flow of LNG in the pipeline, due to the heat input, a part of LNG will be vaporized into gaseous natural gas, which will increase the pressure of the tank and pipeline. In order to ensure the safety of operation and the full utilization of natural gas, the BOG generated by the tank truck, tank and pipeline is collected into the BOG main pipe through the pressure reducing regulating valve and the safety valve, and then heated by the BOG heater and sent to the transmission and distribution network.
(V) Emergency release process
EAG is the abbreviation of Escape Air Gas, which means emergency release of natural gas. All the gases released by the safety valve of the cryogenic system are cryogenic gases. When the temperature is below -113℃, the weight of natural gas is greater than that of air at room temperature. It is not easy to spread when discharged and will accumulate downward. Therefore, it is necessary to set up an air-temperature release gas heater. The released gas first passes through the heater. After heat exchange with the air, the specific gravity of the natural gas will be less than that of the air. After high-point release, it will be easy to spread, so it is not easy to form an explosive mixture near the ground.
2. LNG Bottle Station Gasification Process
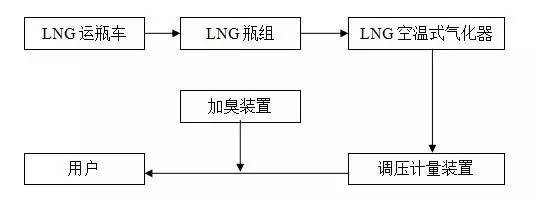
Figure 3 LNG bottle group gasification station process flow
The process flow of the LNG bottle group gasification station is similar to that of the gasification station. The steel cylinder containing liquefied natural gas is transported to the station, and the gas and liquid phase hoses are connected. The steel cylinder is pressurized with the booster provided by the steel cylinder, and the LNG in the steel cylinder is sent to the external air-temperature gasifier by using the pressure difference; the liquefied natural gas is gasified and heated in the gasifier: then the pressure is adjusted to the required pressure by the pressure regulator, and it is sent to the user after metering. The LNG bottle group gas supply process sets up two groups of steel cylinders for use and standby, and the number is the same. When the liquid level of the LNG steel cylinder on the use side drops to the specified liquid level, it should be switched to the standby cylinder group in time, and the empty steel cylinder switched down should also be tanked and used in time. Natural gas itself is colorless and odorless. As a city gas, it should be odorized according to regulations. If the LNG bottle group gas supply process is used in cold areas in the north, there is a heating and temperature-raising device before the natural gas enters the pipeline network, as shown in Figure 3.
Contact Us
Please use the form below to contact us.
If you require a response, we will contact you as soon as possible.

